20 Krf2o Electron Domain Geometry Update

There are 4 electron domains around the central atom p 3 single bonds and 1 double bond.
Krf2o electron domain geometry. 4 bonds in the structure. The electron domain geometry of a boron centered compound bh3 is trigonal planar. A bh 3 there are 3 valence electrons in boron and 1 each in hydrogen so there are 6 electrons total.
32 8 24 e remaining. Draw 4 bonds around c and attach cl to each bond. Electron domain is used in vsepr theory to determine the molecular geometry of a molecule.
The lewis structure is at the right. Geometry is determined by the total number of bonded atoms and lone pairs around the central atom. Ph3 of electron domains electron domain of bonding domains of lone pair domains molecular geometry bond angles present orbital hybridization of this substance polar or non polar.
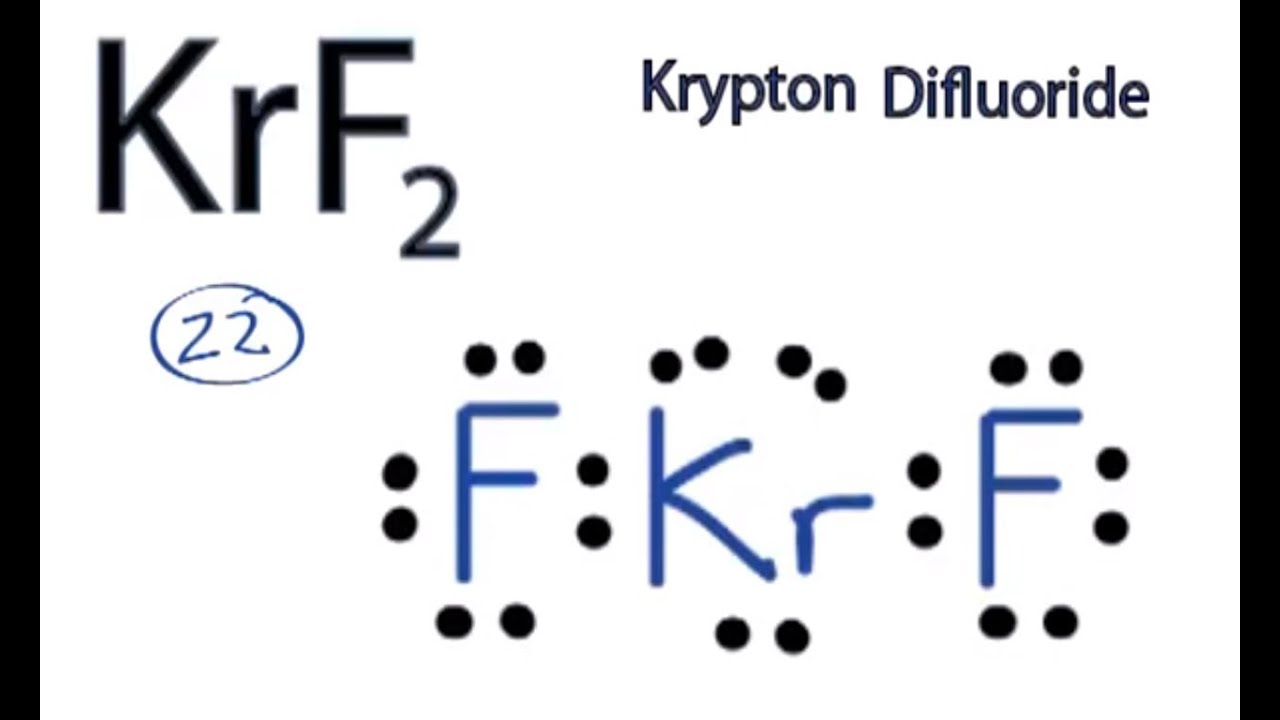
Geometry refers to the bond angles about a central atom. How many valence electrons does krf2 have and what are its electron domain and molecular geometries. The electron domain charge cloud geometry of ici5 s usually positively charged.
This gives an electron geometry or parent geometry of tetrahedral. We use all of them up in drawing the bonds for the molecule. Distribute these to surrounding cls first.
So the lewis structure of krf2 will have a trigonal bipyramid electron pair arrangement with linear molecular geometry. C is the central atom. The geometry of the molecule is determined by the number of bonded atoms plus the number of lone pairs of electrons about the central atom.